How Many Valence Electrons Does Carbon Have? | Physicsread
Neon belongs in the family 8A, also known as 0. This means there are 8 valence electrons in an atom of neon. read more. This closed shell configuration makes neon supremely difficult to oxidize, and difficult to reduce. The inertness, the lack of reactivity, of this Noble Gas, is a function of its electronic...In chemistry and physics, a valence electron is an outer shell electron that is associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed...• Valence electrons • the electrons that are in the highest (outermost) energy level • that level is also called the valence shell of the atom • they are held most loosely. • When scientists draw atoms to show how they chemically bond, they only draw the valence electrons. •Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the 2 s and the 2 p sublevels and so the answer is three. Neon, with its configuration ending in s2p6, has eight valence electrons.The electrons in an atom live in orbits, moving like blindingly fast planets in a cloud around the nucleus. For the transition metals (groups 3-12), figuring out the valence electrons is more complicated. Their atomic structure is such that their d subshell is incomplete.
Valence electron - Wikipedia
Get an answer for 'how many valence electron are in be' and find homework help for other Science questions at eNotes. Valence electron is the generally the electron which is associated with an atom that can participate in the formation of chemical bond.The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction. The term covalent bond is used to describe the bonds in compounds that result from the sharing of one or more pairs of electrons.How many valence electrons does oxygen (O) have? A: Any element in group 1 has just one valence electron. Examples include hydrogen (H), lithium (Li) Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne)...How many valence electrons does neo? Neon has eight valence electrons, a full octet that makes neon quite stable(Noble gas). This can be confirmed via it's electronic configuration by looking at the highest energy level which is 2 in this case.

PDF Valence Electrons
So the maximum number of valence electrons of an atom cannot be more than 8. In this article, we will concentrate on the electrical properties of an element and try to observe how the electrical property is determined by the number of valence electrons in the outermost Neon has 8 Valence electrons.Since neon has 8 valence electrons, a full octet makes neon stable. It is also a noble gas. Then, you do the configuration... This site is using cookies under cookie policy. You can specify conditions of storing and accessing cookies in your browser.The electrons are drawn in a clockwise manner starting on the right. The next electron is drawn Although this is generally the order in which electrons are drawn, it is not the absolute method. Include all of the valence electrons. Hint 1. How to approach the problem First, identify the symbol of...Neon, #Z=10#, has eight valence electrons. This closed shell configuration makes neon supremely difficult to oxidize, and difficult to reduce. How do valence electrons determine chemical reactivity? How many valence electrons are in a silicon atom?Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Since covalent bonds are formed by the sharing of electrons present in the final shell, the number indicates how many bonds are permitted to form.
Yahoo Answers has shut down as of May 4, 2021. Yahoo Answers used to be as soon as a key part of Yahoo's products and services, but it has declined in popularity over time as the desires of our participants have modified. We determined to shift our resources away from Yahoo Answers to focus on merchandise that higher serve our participants and deliver on Yahoo's promise of offering premium depended on content.
As of May 4, 2021 you can no longer access the site, but you'll nonetheless request a obtain of your Yahoo Answers knowledge until June 30, 2021. To lend a hand you with this transition now we have compiled a list of questions that can arise all over this procedure.
Will this have an effect on my Yahoo Account or different Yahoo services and products?No, those adjustments are explicit to Yahoo Answers. They would possibly not affect your Yahoo Account or some other Yahoo services and products.
Where will have to I go when I have questions in the future?Yahoo Search can be utilized to find solutions and information from the web. Our Yahoo COVID page provides info and sources concerning the Coronavirus pandemic.
Can I download my Yahoo Answers content material? What content material is available to me?Your Yahoo Answers information obtain will return all user-generated content, together with your questions, solutions and images. You will be unable to download other customers' content, questions, or answers.
Do I've to download my content?No, the content material obtain is optional. However, if you choose to obtain your content material, you have got to take action sooner than June 30, 2021.
When will I am getting my Yahoo Answers content?Our staff works as rapid as conceivable to make knowledge to be had, but it may possibly take up to 30 days to receive your content download.
I downloaded my Yahoo Answers content material, how do I view it?Your content will probably be formatted in JSON (JavaScript Object Notation) and could be onerous to seem through at a look. We have sources on viewing and managing your account data that help you perceive your knowledge download.
How can I share my comments/comments about this modification?Send any comments or comments you've gotten regarding this decision to yahoo_answers_sunset@verizonmedia.com. Thanks for taking the time to share your ideas with us.
Untitled
Solved: Which Is The Appropriate Electron Configuration Fo... | Chegg.com

Lewis Structure - Homework Help - Science Forums

Beryllium Happy With 4 Valence Electrons

Octet Rule | Easy Hard Science

Valence Electron Practice Worksheet Answers

Untitled
Chemical Bonding: Fundamental Concepts
Valence Electron - Wikipedia
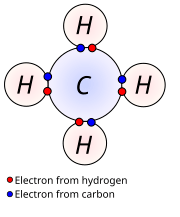
Ions And Isotopes - Docest

Hb Bonding Pogil 2014-2015

CHEM 101: PREPARATORY CHEMISTRY (SP19-ZIMMERMANN) Module 4 Homework Electron Configurations And The Periodic Table Drag The Appropriate Items To Their Respective Bins. View Available Hint(s) Rese... - HomeworkLib

Electron Configuration (Elements 1-20) | Good Science

Valence Electrons | CK-12 Foundation

How Many Electrons Does Fluorine Have? How Many Electron Shells? Name The Orbitals That Are Occupied. How Many Electrons Are Needed To Fill The Valence Shell? - Quora
Neon Element: Chemistry, Element, En, Gas, Glow, Inert, Neon | Glogster EDU - Interactive Multimedia Posters

Electron Configuration For Neon (Page 1) - Line.17QQ.com

Electron Configurations & The Periodic Table

Solved: Ne Neon Has 10 Core Electrons In The N- Level And | Chegg.com

0 comments:
Post a Comment